Acute Ischemic Stroke and Transient Ischemic Attack
- Endovascular treatment of acute ischemic stroke (AIS) enables access to occluded intracranial vessels for local administration of thrombolytics, mechanical embolectomy, and/or angioplasty. There are currently four mechanical devices cleared by the Food and Drug Administration (FDA) for recanalization of arterial occlusion in patients with AIS; however, despite being cleared by the FDA, none of these devices have an FDA clinical indication due to the need for randomized comparison with medical therapy devices. Endovascular interventions are extremely time dependent, and reduced time from symptom onset to reperfusion is highly correlated with better clinical outcomes.
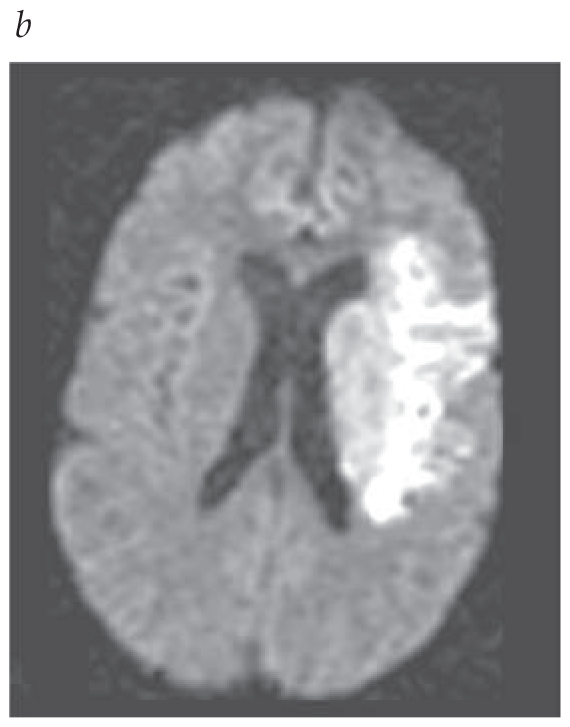
- CTA with CT perfusion or MRA with diffusion-weighted MRI with or without MR perfusion is recommended for certain patients (2019 AHA/ASA recommendation on head imaging).
- 2019 AHA/ASA updates on IV alteplase indications and management in acute ischemic stroke patients.